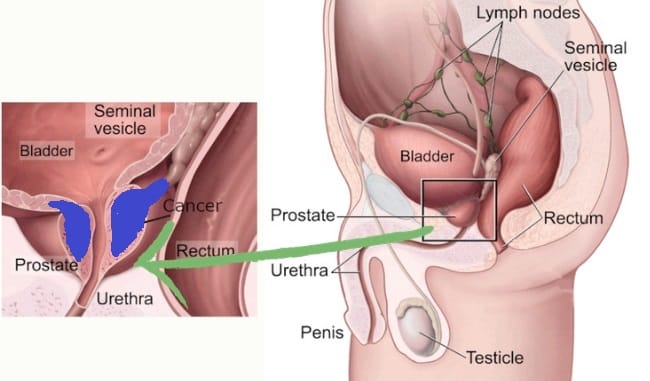
The answers indicate a possibility of regionally advanced prostate cancer, likely corresponding to Stage III. This stage involves TNM values of T3 or T4, where the tumor extends beyond the prostate capsule but without distant spread (N0, M0). In this stage, the cancer may have spread beyond the prostate to nearby tissues or lymph nodes but not to distant sites. Stage III prostate cancer often requires a combination of treatments, including surgery, radiation, and possibly hormone therapy. Consulting with a healthcare provider is crucial to determine the most suitable treatment approach.
NCCN (National Comprehensive Cancer Network) Clinical Staging Guidelines are pivotal in shaping the approach to diagnosing and treating prostate cancer at various stages. The NCCN Guidelines for Prostate Cancer provide a framework for the workup of patients, risk stratification, and management of localized, recurrent, and advanced disease. This includes the management of metastatic castration-sensitive prostate cancer, nonmetastatic castration-resistant prostate cancer (CRPC), and metastatic CRPC. A shared decision-making approach is emphasized, taking into account patient preferences, prior treatments, visceral disease, symptoms, and potential side effects.
Nonmetastatic castration-resistant prostate cancer (nmCRPC)
nmCRPC is characterized by rising prostate-specific antigen (PSA) levels despite castrate levels of testosterone, with ongoing androgen-deprivation therapy (ADT) or orchiectomy, and no detectable metastases by conventional imaging. nmCRPC patients risk progressing to metastatic disease and developing cancer-related symptoms and morbidity. Most nmCRPC patients are asymptomatic from their disease, but they are often older and have chronic comorbidities, necessitating a careful balance of treatment benefits against potential risks. The rationale for early treatment in nmCRPC is to delay metastatic progression and prolong survival. Treatment focuses on second-generation androgen receptor inhibitors (ARIs) such as apalutamide, enzalutamide, and darolutamide. These treatments offer clinical benefits but also come with potential adverse events (AEs) affecting quality of life, physical capacity, and cognitive function.
Apalutamide: FDA-approved for nmCRPC treatment in 2018, apalutamide interacts with the androgen receptor’s ligand-binding domain, inhibiting its activation. In the SPARTAN trial, apalutamide significantly increased median metastasis-free survival (MFS) and overall survival (OS) compared to placebo. However, treatment-emergent AEs like fatigue, rash, falls, fractures, mental impairment, and hypothyroidism were more common with apalutamide than placebo.
Enzalutamide: Enzalutamide was expanded to include nmCRPC treatment in 2018, based on the PROSPER trial. It improved median MFS and OS compared to placebo, but associated AEs included hypertension, mental impairment disorders, and major cardiovascular events. The incidence of AEs leading to discontinuation increased in the enzalutamide arm during the final analysis.
Darolutamide: Structurally distinct from enzalutamide and apalutamide, darolutamide was approved in 2019 for nmCRPC. It showed significant benefits in MFS and OS in the ARAMIS trial. Darolutamide was well tolerated, with low incidences of most ARI-associated AEs compared to placebo.
Effective management of nmCRPC aims to delay metastatic progression while maintaining quality of life. Treatment should ideally begin as early as possible in high-risk patients. However, clinicians must consider the patient’s age, underlying comorbidities, concurrent medications, and symptoms from chronic ADT when initiating treatment.