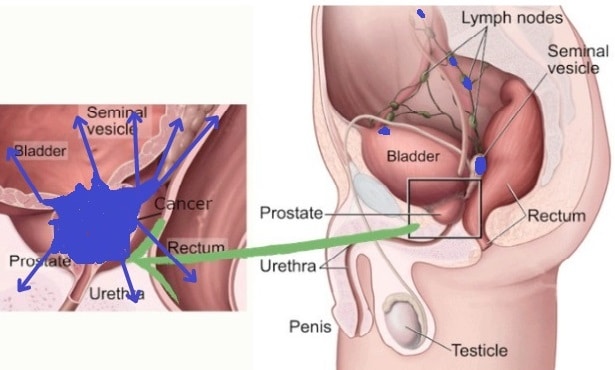
The quiz results suggest advanced or metastatic prostate cancer, likely corresponding to Stage IV. This stage is indicated by any T, N1 (cancer spread to distant lymph nodes), or M1 (distant metastasis), showing significant spread of the cancer. Stage IV cancer management typically involves systemic therapies, and a multidisciplinary medical team is often necessary to address the complexity of treatment. Immediate medical consultation is vital for the most effective care and management.
NCCN (National Comprehensive Cancer Network) Clinical Staging Guidelines are pivotal in shaping the approach to diagnosing and treating prostate cancer at various stages. The NCCN Guidelines for Prostate Cancer provide a framework for the workup of patients, risk stratification, and management of localized, recurrent, and advanced disease. This includes the management of metastatic castration-sensitive prostate cancer, nonmetastatic castration-resistant prostate cancer (CRPC), and metastatic CRPC. A shared decision-making approach is emphasized, taking into account patient preferences, prior treatments, visceral disease, symptoms, and potential side effects.
Metastatic Castration-Sensitive Prostate Cancer (mCSPC)
For mCSPC, Androgen deprivation therapy (ADT) with treatment intensification is strongly recommended. Treatment options include ADT combined with abiraterone, apalutamide, enzalutamide, docetaxel, or external beam radiation therapy (EBRT) for low-metastatic burden. These therapies are preferred for most patients, and ADT alone is generally reserved for patients who cannot tolerate intensive therapy.
| Specific Drug Therapies in mCSPC | The Expected Result |
|---|---|
| Abiraterone Acetate | FDA-approved in combination with prednisone for mCSPC, based on clinical trials demonstrating improved overall survival over ADT alone. |
| Apalutamide | Demonstrated significant improvements in radiographic progression-free survival (PFS) and overall survival (OS) in the TITAN clinical trial, making it a category 1 option for mCSPC. |
| Enzalutamide | Compared with first-generation antiandrogens in the ENZAMET trial, enzalutamide showed improved OS and PFS, supporting its use in mCSPC. |
| Docetaxel | Recommended for patients with castration-sensitive prostate cancer and distant metastases, with studies indicating a longer OS in combination with ADT, especially in high-volume disease. |
| ADT with Docetaxel and Novel Hormone Therapy | The addition of triplet therapies of ADT with docetaxel and novel hormone therapy, either abiraterone or darolutamide, has been included as category 1, preferred options for patients with mCSPC, based on the PEACE-1 and ARASENS trials. |
Metastatic Castration-Resistant Prostate Cancer (mCRPC)
PARP Inhibitors: PARP inhibitors have become significant in treating mCRPC, especially in patients with genomic alterations in the homologous recombination repair (HRR) pathway. The use of olaparib, a PARP inhibitor, has shown improved radiographic progression-free survival and overall survival in patients with specific gene alterations (BRCA1, BRCA2, or ATM) as demonstrated in the phase III PROfound study.
Rucaparib: The TRITON2 study evaluated rucaparib in mCRPC patients with deleterious or somatic mutations in HRR genes who had progressed on prior therapy. This led to the FDA approval of rucaparib for mCRPC patients with BRCA mutations previously treated with a next-generation hormonal agent and taxane.
Cabazitaxel: The CARD trial demonstrated the effectiveness of cabazitaxel as a third-line therapy for mCRPC patients who had progressed on novel androgen receptor–targeted therapy and/or prior docetaxel. This treatment showed improved median progression-free survival and PSA response compared to androgen signaling–targeted inhibitors