Last Updated on July 7, 2024 by Max
Introduction
The question that arises is why we need to understand the mechanism of benign prostatic hyperplasia (BPH). The answer lies in the significance of the mechanism itself – it is the driving force behind everything. If we fail to identify the mechanism of a disease, we can liken ourselves to blind warriors attempting to fight an unseen enemy. We may observe the condition’s symptoms, but our actions are somewhat misguided without understanding the causative process.
In this article, I aim to delve into the mechanism of BPH, first proposed a decade ago by Israeli scientists and corroborated by numerous clinical studies since then.
Understanding the Paradox of Benign Prostatic Hyperplasia
A puzzling aspect of BPH is that, despite age-related declines in circulating testosterone levels, the prostate grows, contrary to expectations. Testosterone is instrumental in promoting the proliferation of muscle cells, including those in the prostate. Interestingly, while its concentration in the circulating blood is relatively low, intraprostatic testosterone and dihydrotestosterone levels and androgen receptor activities remain consistently high.
The prostate, which contributes to about 30% of sperm ejaculate, operates under the control of androgens: free testosterone (FT) and dihydrotestosterone (DHT). The testes produce sperm and free testosterone, the latter of which, along with waste products, is drained into the body’s circulatory system via the internal spermatic veins. However, FT is diluted through venous and arterial circulations, and most of it binds to albumin and sex hormone-binding globulin. Only about 2% of non-bound FT reaches the prostate via the prostate artery. Under normal conditions, FT takes a long journey from the testes through the body’s bloodstream to the prostate. Once there, the enzyme 5α‐reductase transforms approximately 90% of FT irreversibly into DHT, a more potent hormone that more readily associates with the androgen receptors. Numerous evidence-based studies have demonstrated DHT’s critical role in the pathology of BPH.
The Link Between Varicocele and Benign Prostatic Hyperplasia
Gat et al. found that the blood drainage of the reproductive system is not working correctly due to the damage of the one-way valves in the internal spermatic veins. Destruction of one‐way valves is a well-known vascular disease, varicocele, progressing with age and reaching over 75% at the age of 70.
Several independent studies on hundreds of patients showed the presence of varicocele in all cases with BPH. Moreover, there is a high correlation between varicocele at the ages of 20-25 years and the development of BPH in men of 45 years.
Some speculate that this occurrence is the price we pay for the human’s advantageous erect posture. While our erect posture has allowed for greater hand function and creativity, it has also resulted in blood pressure-related diseases, back pain, varicocele, and BPH. Gat et al. have also linked the destruction of the testicular venous drainage system with almost 90% of male infertility cases.
Whether BPH is a normal consequence of an erect posture in aging men is debatable. Other significant factors such as sexual excesses, lifestyle choices, obesity, environmental pollution, and diet could trigger the process of varicocele. Additionally, this theory contradicts the findings of Jean D. and Wilson M.D., published in “The American Journal of Medicine”: “Development of prostatic hyperplasia is an almost universal feature of the aging man and dog, and in both species, the process develops only in males with intact testes.” However, it is noted that in dogs, the deferent duct (DV) opens into the prostatic venous system, allowing a significant concentration of androgens to enter the prostatic circulation. No valves have been found in these vessels, which might result in elevated hydrostatic pressure in the testicular drainage system when the dog sits. However, other factors, including the shorter lifespan of dogs compared to humans, may also influence prostate pathology.
The Underlying Mechanism of Benign Prostatic Hyperplasia
With the one-way valves in the internal spermatic vein damaged, the vein can no longer effectively serve as a drainage system. Elevated hydrostatic pressure from a column of blood in the vein leads to:
- Persistent hypoxia in the testicular microcirculation results in degeneration in spermatogenesis and a decrease in sperm production.
- Redirection of undiluted FT flow straight from the testes into the prostate. The venous blood cannot flow upwards and is diverted into other channels common with the prostate. Hence, in addition to receiving physiological FT from the arterial blood, the prostate becomes inundated with undiluted androgen, around 100 times above the normal level, from an unexpected source.
This sudden influx of undiluted androgen accelerates prostate cell production and prolongs cell lifespan, contributing to the development of BPH. These observations have been clinically proven in a large number of patients. A recent study (Goren M, Gat Y. 2018) observed bilateral varicocele in all 901 patients. Sclerotherapy of the spermatic vein reduced prostate volume in over 80% of men, with a significant improvement in prostate symptoms. This study establishes a clear pathophysiological link between bilateral varicocele and BPH.
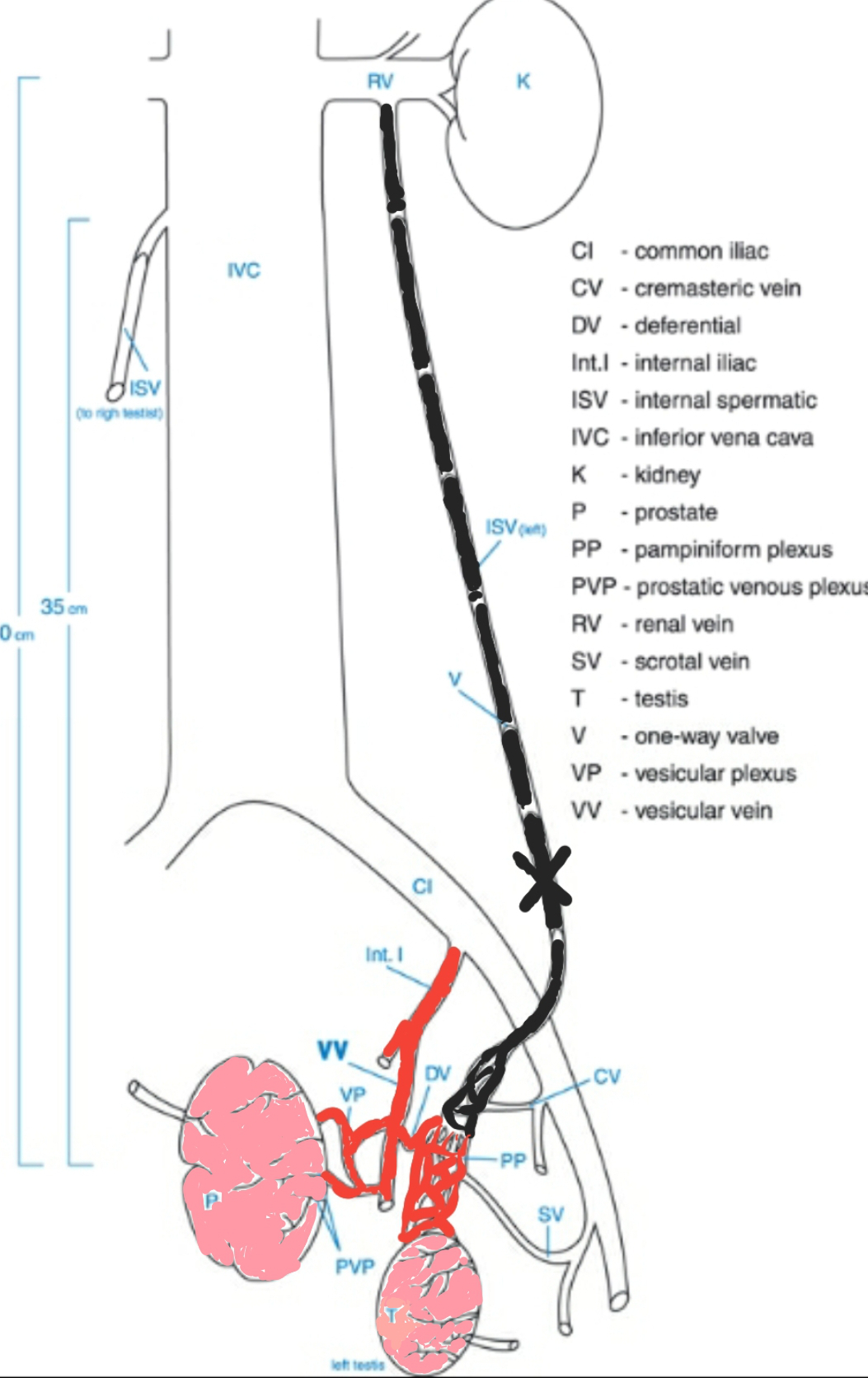
This accelerates prostate cell production and prolongs cell lifespan, leading to the development of BPH.
Furthermore, these observations were clinically proven in a large number of patients. A recent study (Goren M, Gat Y. 2018 ) observed bilateral varicocele in all 901 patients. Sclerotherapy of the spermatic vein reduced prostate volume in more than 80% of men, with prostate symptoms improved. A straightforward pathophysiologic connection exists between bilateral varicocele and BPH.
In the figure, the varicocele-affected spermatic vein is shown black, and the vessels common for testicles and prostate are shown red.
The Potential Medical Consequences
Reflecting on the possible medical implications of these scientific discoveries, the editor-in-chief of the “Andrology” journal, W. -B. Schill, stated: “The study by Gat et al. has several potentially powerful medical consequences. Firstly, the savings in hospitalizations and surgical and indirect costs would be substantial. Secondly, if varicocele predisposes to later-age prostatic pathology, the best strategy would be to establish criteria for preventive treatment by interventional radiology. Thirdly, their treatment restored circulating testosterone levels in aging men and could ‘naturally’ revive sexual function.”
Considering these findings, BPH should no longer be viewed as a paradoxical condition in aging men; rather, it should be expected. We now better understand the mechanism of benign prostatic hyperplasia, bringing us a step closer to defeating this common affliction.
For those concerned about their health and sexual well-being, I would strongly recommend consulting with a medical professional about varicocele therapy’s benefits and potential downsides. Once the main issue has been addressed, the focus can be shifted toward maintaining prostate health through a balanced diet and healthy lifestyle choices.
BPH: Recent Discoveries
Let’s talk about some mind-blowing research from Nature Communications. These guys dived deep into BPH’s molecular mechanics and discovered two distinct subtypes of BPH, each with unique molecular signatures. This discovery could lead to precision therapies tailored for each subtype. In particular, they found that mTOR inhibitors, a class of drugs typically used in cancer treatment, could be a new candidate for treating one of these BPH subtypes.
You may thought BPH was just an unfortunate side effect of getting old. A study in Scientific Reports suggests that 60% of the variation in BPH is down to genetics. So it looks like, your genes could be setting you up for a future of BPH woes. They even found a specific gene, SYN3, on chromosome 22 linked to BPH risk. So maybe one day, you could take a DNA test to discover your BPH destiny.
Then, let’s add another layer to this mystery. A study published in Cell Death Discovery found that the Smoothened (SMO) cascade, a key player in the Hedgehog signaling pathway, which normally helps with organ growth and maintenance, is involved in BPH. Blocking this pathway could reduce cell proliferation and tissue fibrosis in BPH. In other words, jamming this molecular signal could be a new way to put the brakes on BPH.
So, next time you think about BPH, remember it’s not just about an aging prostate. We’re discussing a complex interplay of molecular subtypes, genetic risk factors, and runaway signaling pathways. And who knows? With all this cutting-edge research, a breakthrough treatment can be just around the corner.
References
- W. -B. Schill I. Ben‐Shlomo. Benign prostatic hyperplasia due to venous drainage malfunction in the male reproductive system: a price of erect posture in humans. Andrologia 2008 Volume 40, Issue 5 P: 272
- Y. Gat M. Goren. Benign Prostatic Hyperplasia: Long‐term follow‐up of prostate volume reduction after Sclerotherapy of the internal spermatic veins. Andrologia. 2018 Mar;50(2)
- Canales, Benjamin K. et al. Prevalence and effect of varicoceles in an elderly population.
Urology, 2005 Volume66, Issue3, 627 – 631 - Y. Gat M. Gornish M. Heiblum S. Joshua. Reversal of benign prostate hyperplasia by selective occlusion of impaired venous drainage in the male reproductive system: novel mechanism, new treatment. Andrologia 2008 Volume 40, Issue 5.
- Jean D.WilsonM.D. The pathogenesis of benign prostatic hyperplasia.The American Journal of Medicine. Volume 68, Issue 5, May 1980, Pages 745-756
- Goren M, Gat Y. Varicocele is the root cause of BPH: Destruction of the valves in the spermatic veins produces
elevated pressure, which diverts undiluted testosterone directly from the testes to the prostate. Andrologia. 2018 Jun;50(5) - Liu, D., Shoag, J.E., Poliak, D. et al. Integrative multiplatform molecular profiling of benign prostatic hyperplasia identifies distinct subtypes. Nat Commun 11, 1987 (2020). https://doi.org/10.1038/s41467-020-15913-6
- Hellwege, J.N., Stallings, S., Torstenson, E.S. et al. Heritability and genome-wide association study of benign prostatic hyperplasia (BPH) in the eMERGE network. Sci Rep 9, 6077 (2019). https://doi.org/10.1038/s41598-019-42427-z
- Liu, J., Yin, J., Chen, P. et al. Smoothened inhibition leads to decreased cell proliferation and suppressed tissue fibrosis in the development of benign prostatic hyperplasia. Cell Death Discov. 7, 115 (2021). https://doi.org/10.1038/s41420-021-00501-4
